Physical Chemistry in a nutshell
The Basics of Thermodynamics, Kinetics and Electrochemistry for Engineers and Scientists
Reiter

Physical Chemistry in a Nutshell
Welcome to Physical Chemistry in a nutshell
PhysicalChemistry in a nutshell is a one-semester course on the basic knowledge of physical chemistry relevant to science-oriented engineers.
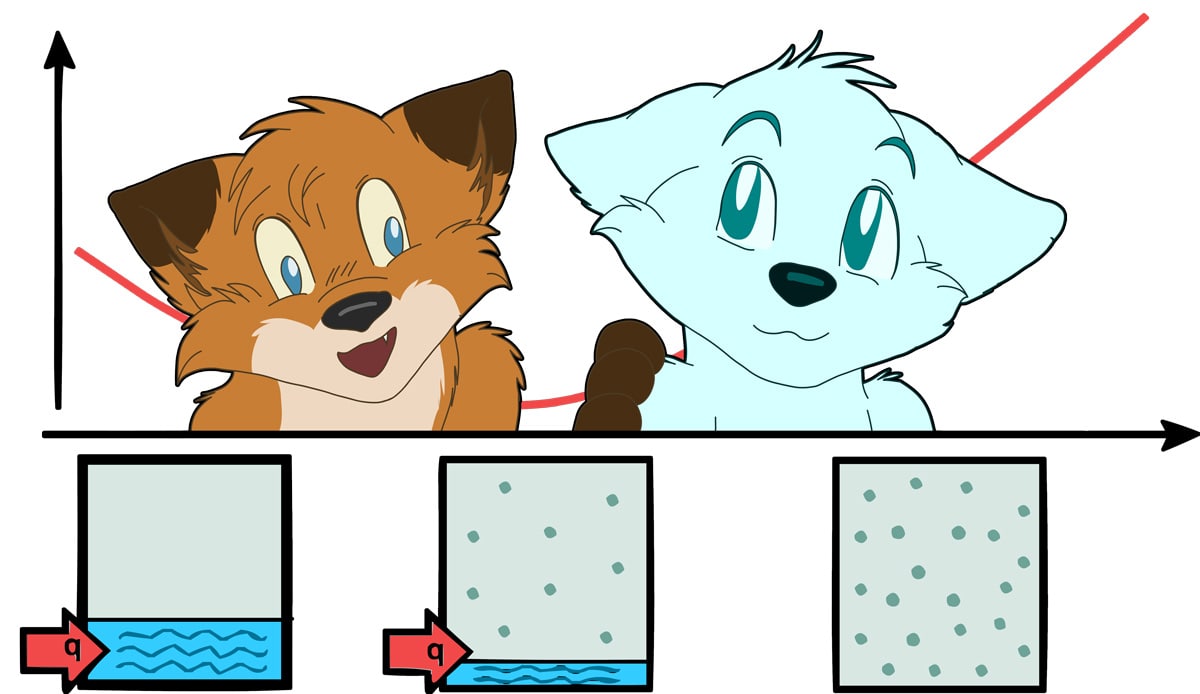
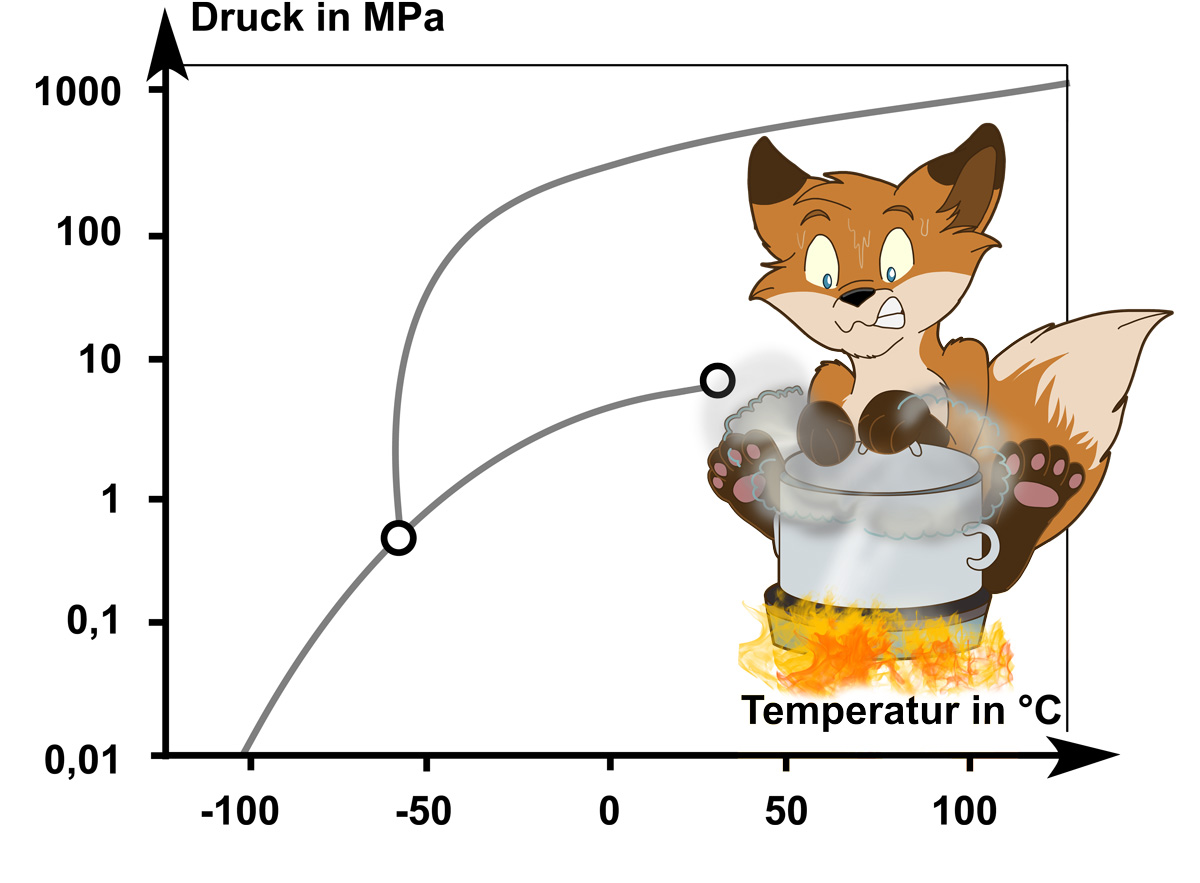
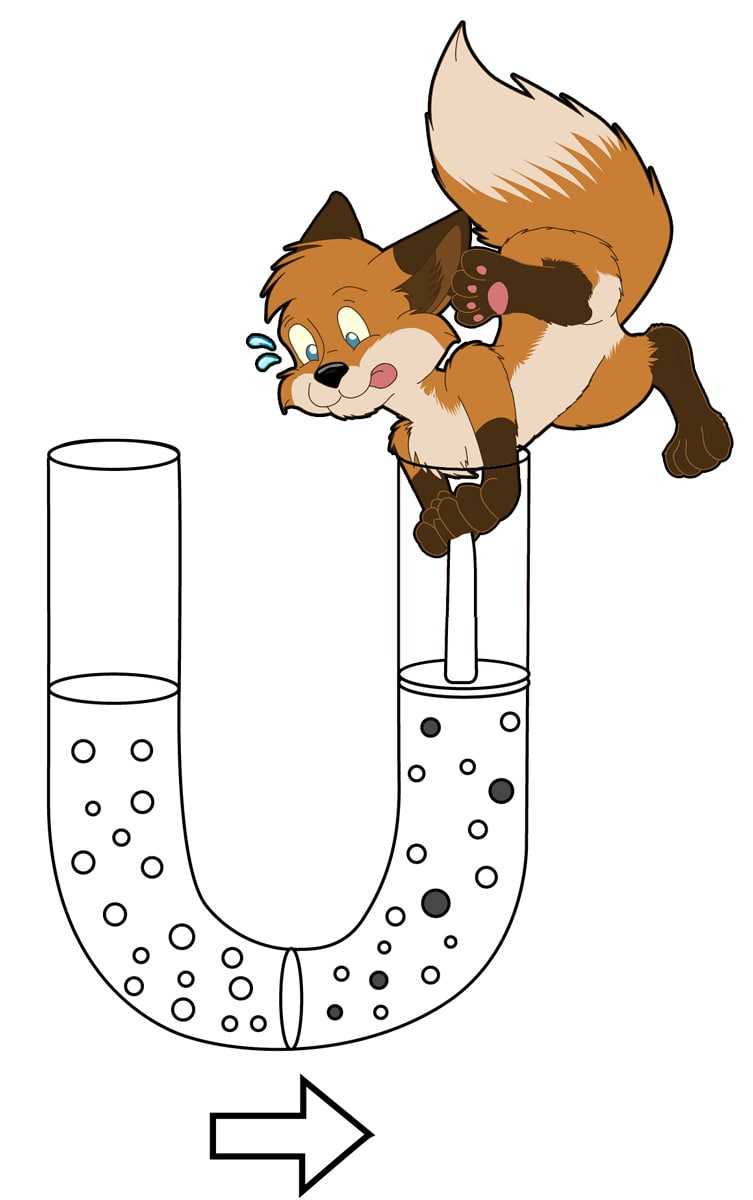
It covers the fundamentals of chemical thermodynamics, phase equilibria, reaction kinetics and electrochemistry.
It covers the fundamentals of chemical thermodynamics, phase equilibria, reaction kinetics and electrochemistry.
PhysicalChemistry in a nutshell consists of 12 "lecture bites" (chapters / learning units)
There are multimedia materials for each lecture bite (handout, literature, videos, ILIAS tests, exercises)
as well as at least one live event (video meeting, workshop, lecture, tutorial).
The live events at Campus Jülich only take place in summer semester.
The videos for the course are available both in English and German on the AV portal of the Technical Information Library (TIB) and on YouTube.
There are multimedia materials for each lecture bite (handout, literature, videos, ILIAS tests, exercises)
as well as at least one live event (video meeting, workshop, lecture, tutorial).
The live events at Campus Jülich only take place in summer semester.
The videos for the course are available both in English and German on the AV portal of the Technical Information Library (TIB) and on YouTube.
Textbooks
Textbooks
The course is based on the textbook "Physical Chemistry in a nutshell"
The textbook "Chemistry - the central science" by Brown / LeMay is also recommended.
The classic textbooks by Engel / Reid and Atkins / dePaula, in which the entire field of physical chemistry is summarized, are suitable as reference works.
How to work the learning units
1.) the handout provides an overview
2.) The videos offer an introduction to the topic
4.) Exercises and Quizzes serve to control learning.
Performing the online exercises is limited to 3 times.
You must score at least 50% of the possible points to pass the course.
Performing the online exercises is limited to 3 times.
You must score at least 50% of the possible points to pass the course.

5.) Questions can be asked in the forum

6.) In the live event, the topic is deepened interactively; Forum questions are answered and important exercises are discussed
Some pdf files are password protected. The password is a short combination of letters and numbers and can be found under the link.
Lecture Bites (learning units)
The learning units will be activated in the course of the winter semester (odd semester).
artwork by FaelisPawArtistry